Page 55 - RPIA_26-3
P. 55
HYPERSENSITIVITY TO NONSTEROIDAL ANTI-INFLAMMATORY DRUGS:
FROM PATHOGENESIS TO CLINICAL PRACTICE / ARTIGO DE REVISÃO
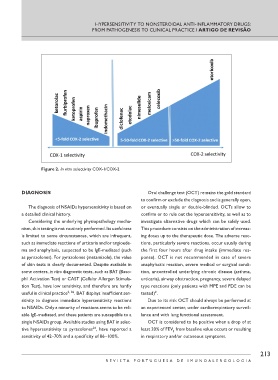
Figure 2. In vitro selectivity COX-1/COX-2
DIAGNOSIS Oral challenge test (OCT) remains the gold standard
to confirm or exclude the diagnosis and is generally open,
The diagnosis of NSAIDs hypersensitivity is based on or eventually single or double -blinded. OCTs allow to
a detailed clinical history. confirm or to rule out the hypersensitivity, as well as to
Considering the underlying physiopathology mecha- investigate alternative drugs which can be safely used.
nism, skin testing is not routinely performed. Its usefulness This procedure consists on the administration of increas-
is limited to some circumstances, which are infrequent, ing doses up to the therapeutic dose. The adverse reac-
such as immediate reactions of urticaria and/or angioede- tions, particularly severe reactions, occur usually during
ma and anaphylaxis, suspected to be IgE -mediated (such the first four hours after drug intake (immediate res-
as pyrazolones). For pyrazolones (metamizole), the value ponse). OCT is not recommended in case of severe
of skin tests is clearly documented. Despite available in anaphylactic reaction, severe medical or surgical condi-
some centers, in vitro diagnostic tests, such as BAT (Baso- tion, uncontrolled underlying chronic disease (asthma,
phil Activation Test) or CAST (Cellular Allergen Stimula- urticaria), airway obstruction, pregnancy, severe delayed
tion Test), have low sensitivity, and therefore are hardly type reactions (only patients with MPE and FDE can be
useful in clinical practice 2, 26 . BAT displays insufficient sen- tested) .
2
sitivity to diagnose immediate hypersensitivity reactions Due to its risk OCT should always be performed at
to NSAIDs. Only a minority of reactions seems to be reli- an experienced center, under cardiorespiratory surveil-
able IgE -mediated, and these patients are susceptible to a lance and with lung functional assessment.
single NSAIDs group. Available studies using BAT in selec- OCT is considered to be positive when a drop of at
tive hypersensitivity to pyrazolones , have reported a least 20% of FEV from baseline value occurs or resulting
26
1
sensitivity of 42–70% and a specificity of 86–100%. in respiratory and/or cutaneous symptoms.
213
REVIST A POR TUGUESA DE IMUNO ALERGOLOGIA